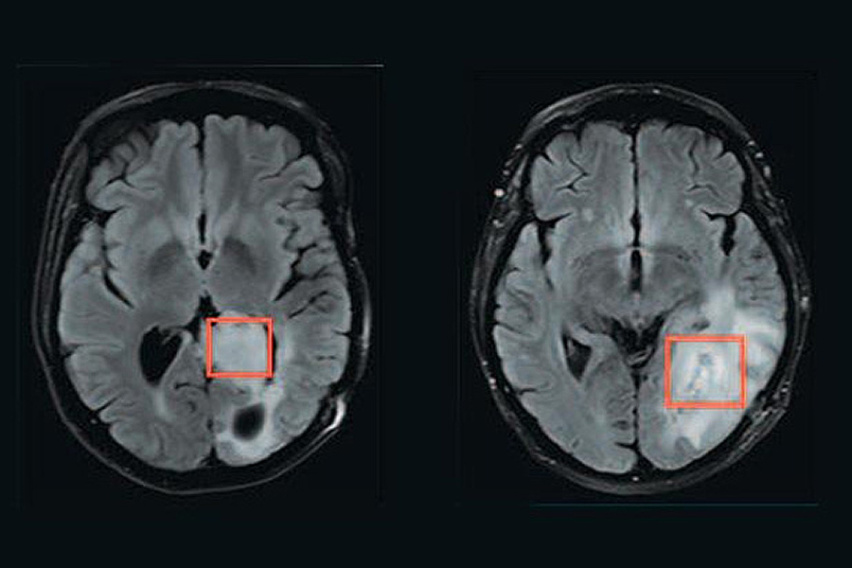
Using magnetic resonance spectroscopy, a team of researchers has developed a way to measure whether brain tumors have a mutation in a gene called IDH. The tissue being analyzed is inside the red boxes. The tumor on the left has the mutation, while the tumor on the right does not.
MIT Koch Institute
June 1, 2023
At the most recent American Society for Clincial Oncology (ASCO) meeting, clinical researchers announced positive results from Phase 3 trials of a new therapy—which leverages research involving Matthew Vander Heiden—for low-grade IDH-mutant glioma, a type of brain cancer. The first therapeutic breakthrough in this disease in more than 20 years, early use of the drug significantly reduced the risk of disease progression or death.
IDH is a metabolic enzyme that when mutated produces an oncogenic metabolite called 2HG that accumulates to high levels in cancer cells and Vander Heiden’s work contributed to this discovery. He has also worked with collaborators via the Bridge Project to assess 2HG non-invasively in patients as a biomarker, and for use in imaging-based diagnostic approaches, helping to lay the groundwork for mutant IDH as a therapeutic target.
Vander Heiden’s brain cancer work took a personal turn when Steven Keating, SM ’12, PhD ’16, a graduate student in the Department of Mechanical Engineering, sought out Vander Heiden to discuss his work. Keating had recently been diagnosed with IDH-mutant brain cancer, an astrocytoma that would claim his life a few years later. In true MIT fashion, Keating approached his tumor as a learning opportunity: he used his brain scans to create 3D-printed models of his own tumor and stimulate discussions on patient data. Further, before and after his tumor removal surgery, he underwent the scanning technique developed by Vander Heiden and his collaborators to monitor changes in 2HG levels. Although the scanning technique was successful, at that time there was no treatment available for Keating’s IDH-mutant brain cancer.
Beyond the direct promise of these clinical trial results for glioma patients, they also suggest potential new applications for Vander Heiden’s research and the diagnostic techniques he developed with his clinical collaborators. Not only could the scan he developed be used to assess which patients might benefit from receiving the new drug, but also to monitor treatment response. Moreover, if the drug is found to be effective in other IDH-mutant cancers—such as gall bladder cancer—its effect could be even more far reaching.

